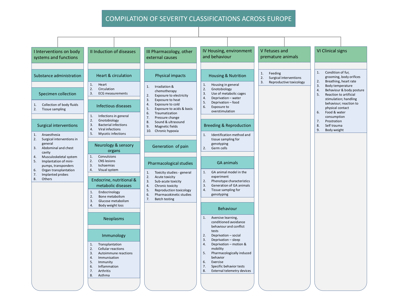
This section gives an overview of systems currently in use in Europe for severity classification of techniques and procedures, and for genetically altered (GA) laboratory animals. As an aid to the efforts being made to harmonise severity classification, we have collected existing official guidelines and and compiled their contents in tables, making it easier to compare them.
To avoid any misunderstandings, and to make the tables more user-friendly, we have also included examples of clinical signs.
An overview of the compilation is shown below (click on the chart to enlarge):
The compilation is, at present, based on catalogues of severity classifications in mammals. As the structure of available guidelines differs significantly, partial duplication was necessary to address all the categories in our compilation.
The compilation covers
- Interventions on body systems and functions
- Induction of diseases
- Pharmacology and other external causes
- Housing, environment and behaviour
- Foetuses and premature animals
- Clinical signs
The background for this compilation:
Severity classification is an important factor in the project authorisation of animal experiments and mandatory according to Directive 2010/63/EU. The assignment to a respective severity category needs careful evaluation of the impact on the animals well-being. To facilitate a common understanding, and avoid decisions on a subjective basis, several guidelines are available on how experimental interventions could be classified. The guidelines are in use by scientists, Project Evaluation Committees and Animal Welfare Bodies. It is important to note that experiment-specific conditions, such as cumulative severity, setting early end-points or other refinements must be taken into account on a case-by-case basis, and may modify the recommended severity classification.
The following publications assist with the process of classifying procedures and experiments, and give examples to facilitate the evaluation process related to multiple-step procedures:
- European Commission (2013): Examples to illustrate the process of severity classification, day-to-day assessment and actual severity assessment. Last updated on 11.01.2013. Available in the EU Commission guidance document on severity assessment framework (from page 20).
- Smith, David; Anderson, David; Degryse, Anne-Dominique; Bol, Carla; Criado, Ana; Ferrara, Alessia et al. (2018): Classification and reporting of severity experienced by animals used in scientific procedures. FELASA/ECLAM/ESLAV Working Group report. In: Laboratory Animals, 52 (1_suppl), 5–57. doi: 10.1177/0023677217744587.
This work is the result of a collaboration between Anne Zintzsch (ICAR3R, Giessen), to whom comments should be sent; Jan-Bas Prins (Leiden University Medical Centre and the Francis Crick Institute); Nicolaos Kostomitsopoulos (Centre of Clinical, Experimental Surgery and Translational Research, Biomedical Research Foundation of the Academy of Athens); and Adrian Smith (Norecopa).
A poster presentation at the 2019 FELASA congress can be downloaded here.
Thanks for your feedback! Please note that we cannot reply to you unless you send us an email.
What are you looking for?
We value your feedback so we can improve the information on the page. Please add your email address if you would like a reply. Thank you in advance for your help.!
Please contact us by email if you have any questions.