|
Newsletter no. 4-2025 from Norecopa
Welcome to Norecopa's fourth newsletter of 2025. This is the 129th newsletter which we have issued.
We hope you find them of use! We
welcome feedback, positive or negative.
Please share this newsletter with your colleagues and friends, and encourage them
to subscribe!
We are also on
LinkedIn and (to a much lesser extent) on
Facebook.
This newsletter contains the following items (if some links do not appear to work, check that your mail program has opened the whole of the newsletter):
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
|
The summer vacation is definitely over for most, and Norecopa's
Webinars & Meetings Calendar bears witness to this.
Much of August has gone to activities "behind the scenes" (manuscripts, committee work, interviews and updating Norecopa's website), but two events under preparation should be mentioned:
• Norway's National Committee are arranging an online meeting on
best practice in wild terrestrial mammal research on 14 October.
• Norecopa's 18th Birthday on Friday 10 October. There will be a link in
Norecopa's Calendar to more information shortly.
Norecopa's Secretary served on the Scientific Committee of the
13th World Congress on Alternatives and Animal Use in the Life Sciences, which took place outside Rio de Janeiro from 31 August to 4 September. He was also first author of two oral presentations and second author of a third. These presentations (and video recordings of two of them) can be downloaded here:
• The Path to Better Science: Practical advice on implementing the 3Rs and related principles in animal research (Adrian Smith, Elisabeth
Pagels & Øyvind Wærenskjold) (pdf) (video)
• 3R alternatives in the education and training of lab animal personnel (Adrian Smith) (pdf) (video)
• Overview about recent European 3R activities with a focus on 3R Centres and the EU Cost Action IMPROVE (Jeffrey Bajramovic, Adrian Smith, Anna Olsson, Arti Ahluwalia
& Winfried Neuhaus) (pdf)
The complete programme of the Congress
is available here.
Adrian Smith had to cancel his physical attendance at the Congress at relatively short notice due to illness, and delivered his presentations as video recordings. Using the money saved on travel and accommodation, Norecopa then donated $1,250 to the Congress to enable early career researchers in the vicinity of Rio, who could not afford the full registration fee, to purchase day
attendance tickets at $50 each.
The next World Congress will be in
Seoul, on 15-19 August 2027.
|
|
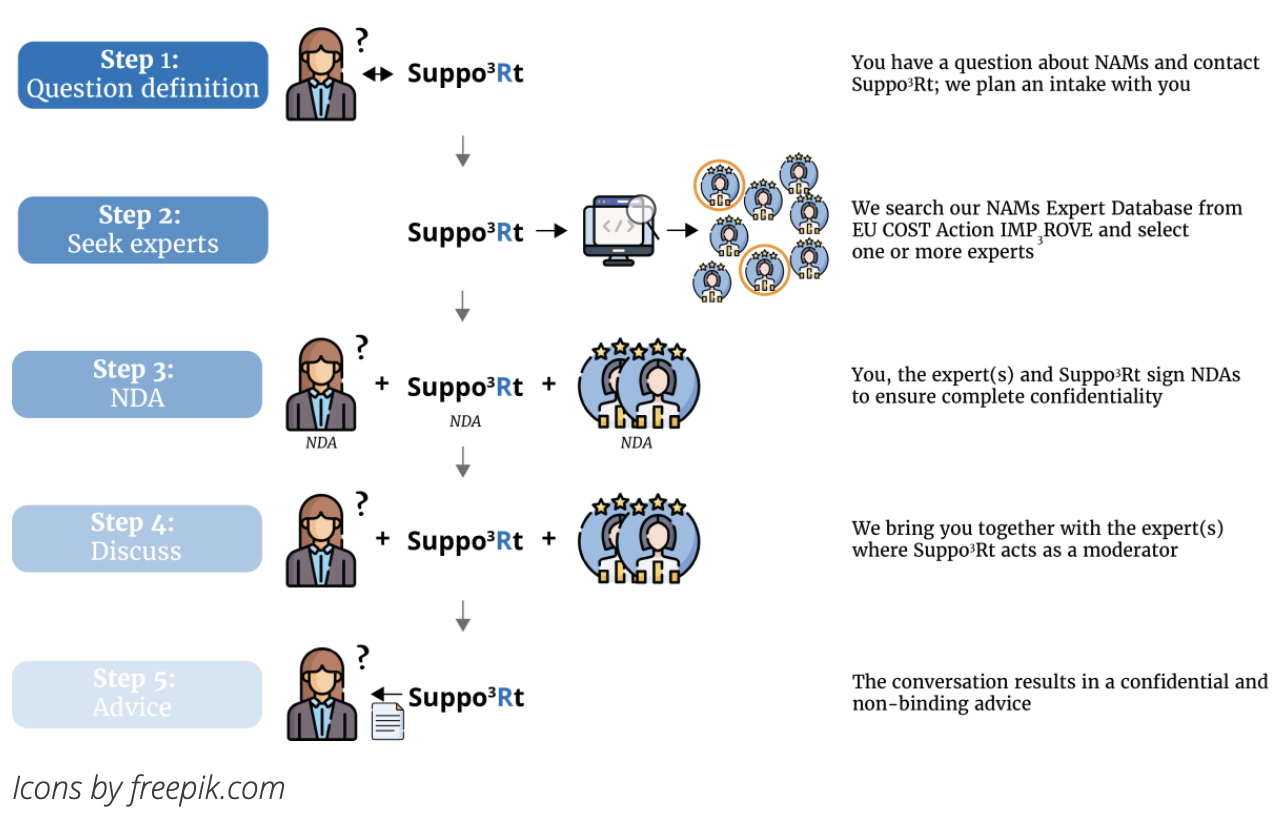
The 3Rs Centre in Utrecht has established
a Support Desk, Suppo3Rt, for those seeking information on NAMs (New Approach Methodologies).
Denmarks 3R-Center is arranging its popular
annual symposium in Copenhagen on 12-13 November. Free registration, with the possibility of submitting a poster, is open until
29 October.
Starting this year, the Centre will be introducing
a 3Rs Talent Award for early career scientists, which will supplement their annual 3R prize. In addition, they are again inviting applications for
3R funds from their pool of DK 1.5 million. The deadline is
8 October. Researchers affiliated with organisations, institutions or businesses in Denmark can apply. Researchers whose main affiliation lies outside of Denmark will not be considered as principal applicants, but can be included as collaborators.
The RSPCA and UFAW are hosting the 32nd Rodent Welfare Group Meeting at the University of Edinburgh on 7 October. One of the key themes this year is home cage monitoring. The closing date for
registration is
28 September.
The UK Animals in Science Committee has published a useful
collection of research reports, covering a range of topical issues such as use of the forced swim test, harm-benefit analysis, antibody licences and non-human primates bred for scientific purposes.
The Swedish 3R centre has translated its
advice on refined methods for blood sampling into English.
The
Veterinary Clinical Skills and Simulation Group may well be of interest to many working with animals for scientific purposes.
Animal Research Tomorrow (ART) has announce its call for the
2026 SciComm and 3Rs ART Awards. The deadline for submission is
16:00 CET on 31 October.
Would you like to direct the Finnish 3R Centre's activities within Replacement, Reduction and Refinement? The Centre is seeking
three new directors for 3-year terms of office. The deadline for applications is
15 September.
The
Animal Welfare Institute (AWI) is accepting applications for two refinement funding opportunities:
•
The Refinement Research Award, which provides up to US$15,000 to fund a research project aimed at developing or testing innovative methods of refinement to the husbandry, handling, or housing of animals used in experimentation to improve their welfare; and
• The Implementing Refinement Grant, which provides up to US$8,000 toward the implementation of a refinement (e.g., purchase of equipment or staff training) that will improve the welfare of animals used in experimentation.
The deadline to submit an application is
23:59 ET on 13 October. These and other funding opportunities
are described here.
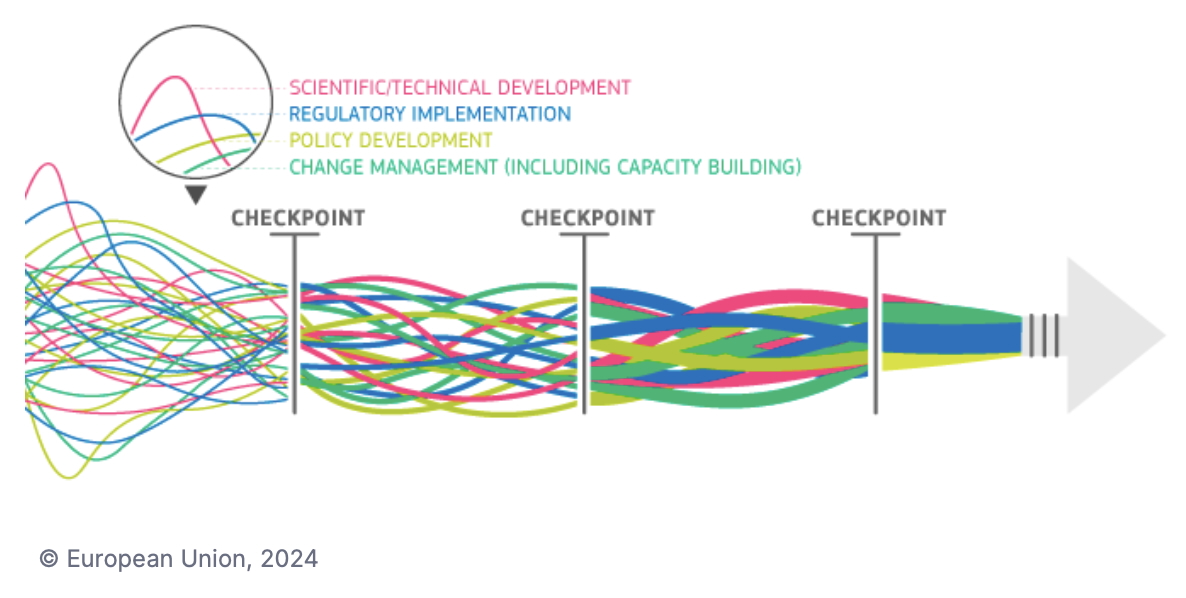
The COST Action
IMPROVE, of which Norecopa is a member, invites scientists who are experts on NAMs (New Approach Methodologies) to register themselves in a
NAM Expert Pool Database. The database will be governed by the management team of EU COST IMPROVE and is not public. 3R Centres participating in IMPROVE can have access to the database upon request, and may use the information to make contact with third parties for intermediary purposes.
Due to maintenance work at the building site, the next EUSAAT conference will not be
held until
17-19 February 2027, in Linz as usual.
The Dutch organisation
Proefdiervrij, which supports scientists that advance the development of innovative alternatives to animal testing, will
from 2026 no longer fund projects that use Fetal Calf Serum.
Thomas Hartung and colleagues have published
draft guidance for Good In Vitro Reporting Standards (GIVReSt). It is for stakeholder discussion and backgound documentation. This may be compared with
the RIVER recommendations developed by an international working group.
For those not already aware of it:
the Refinement Database is a curated collection of published scientific articles and books on topics related to the refinement of housing, husbandry, care, and use of animals in research and testing. The database is intended to provide the most up-to-date information on best practices for improving or safeguarding animal welfare. It is updated every four months; the most recent update was made in July 2025.
The Belgian
RE-PLACE project has produced a video on
How to Search for Alternatives to Animal Testing. The site is well worth searching for alternative techniques, for example within
education and training and
translational research.
The
NAMWISE project aims to drive the adoption and integration of NAMs for assessing the safety and efficacy of chemicals and pharmaceuticals. By delivering recommendations, guidance, and training resources, NAMWISE aims to pave the way toward effective validation and implementation of NAMs.
Subscribe to their newsletter here. They have produced several
videos about the project.
The University of Applied Science and Arts in Northwestern Switzerland will be starting an
advanced course on implementation of the 3Rs, worth 10 ECTS points, on 22 January 2026, with
introductory webinars on 25 September and 26 November.
|
|
A so-called
EU Commission Delegated Directive (2024/1262) issued on 13 March 2024 amends
Directive 2010/63/EU by issuing new requirements for establishments, the care and accommodation of animals, and the methods of killing animals. Annex III (Requirements for establishments and for care and accommodation of animals) and Annex IV (Methods of killing animals) have been amended.
At the time of the adoption of the Directive, insufficient scientific evidence was available on the
appropriate housing and care requirements for certain species, including cephalopods, zebra fish and passerine birds, and on the appropriate killing methods for cephalopods. Therefore, no species-specific requirements were included in Annex III to Directive 2010/63/EU for those species or for the killing of cephalopods in Annex IV to that Directive.
Some of the new requirements identified for zebra fish and cephalopods that were not included in Annex III to Directive 2010/63/EU should
be introduced for all aquatic species or for all animals.
The information submitted under Article 54(3) of Directive 2010/63/EU shows that several Member States consider hypothermic shock as an appropriate method of killing for zebra fish based on the current scientific evidence. To avoid unnecessary administrative burden arising from regular exemptions granted under Article 6(4)(a) of Directive 2010/63/EU, this method should be allowed for killing of zebra fish.
Since the
adoption of Directive 2010/63/EU, new scientific evidence has emerged on the inappropriateness of using inert gases (argon and nitrogen) to kill rodents, and therefore their use should no longer be allowed for the killing of rodents.
Member States (and Norway who has transposed the Directive) are required to adopt and publish, by
4 December 2025 at the latest, the laws, regulations and administrative provisions necessary to comply with this Directive. The new provisions must be practised by 4 December 2026.
|
|
Free registration is open for
AAALAC's international conference, with a focus on Empowering a
Culture of Care, on 7-8 October. Abstracts of the presentations
can be read here.
Applications are also invited for two awards: AAALAC's International Fellowship recognises two outstanding individuals - one registered by AALAS (USA) or CALAS (Canada) and one by the IAT (UK), who have made (or have the potential to make) significant contributions to the field of laboratory animal care and use. The AALAS/CALAS winner will receive a week-long guest visit to prestigious
biomedical research facilities in the U.K. and complimentary attendance at the U.K.'s largest laboratory animal science and technology meeting, with all registration, travel, lodging, meals, and out-of-pocket expenses covered. The IAT winner will receive a week-long guest visit to prestigious biomedical research facilities in the U.S., plus complimentary attendance at the National AALAS Meeting and similarly with expenses covered.
Application can be made online.
|
|
A short interactive
e-learning course module on preregistration of animal studies was launched at the World Congress in Rio.
Written by Julia Menon of
Preclinicaltrials.eu and Nuno Henrique Franco of
ETPLAS and
i3S, University of Porto, it can be integrated into larger courses and training programs.
Quoting from the abstract of their
presentation at WC13:
Preregistration, the act of publicly registering a detailed research protocol before starting a scientific study, is a mandatory step for clinical trials, and it has recently gained traction in animal-based biomedical research.
Preregistering research protocols helps improve accountability and transparency by sharing in advance the hypotheses, study design, and
analysis plan. Moreover, it serves reproducibility and integrity by describing details that might go missing in manuscripts, and by reducing the risk of reporting biases, and questionable research practices such as p-hacking and HARKing (Hypothesizing After Results are Known). It also furthers the 3Rs principle of Reduction by contributing to avoid involuntary duplication of studies and by making all studies available, including interrupted or unpublished ones.
Despite its benefits, the
adoption of preregistration remains limited in biomedical research. Barriers include lack of awareness, misconceptions about the method (e.g., perceived administrative burden, concerns about scooping and lack of scientific creativity), and challenges in integrating new topics into already packed curricula. As a result, preregistration is not incorporated into 3Rs education globally, limiting its potential to improve research quality and reduce unnecessary animal use.
Those who are
interested in using or embedding this module, or in learning more about pre-registration, are
invited to use this form.
|
|
Various techniques have been developed to reduce stress associated with handling mice, not least when changing cages.
Harikrishnan Vijayakumar Sreelatha and colleagues have designed and developed
the "Mus-Mobile", a box that can be used to move up to 5-6 mice simultaneously from cage to cage.
They have produced a video of the procedure, which they invited Norecopa to publish on
its Vimeo channel.
They plan to make the device commercially available soon via the Indian cage manufacturer Citizen Industries in Ahmedabad.
|
|
The European Commission has decided to establish a dynamic catalogue of 'transitional initiatives', which are planned courses of action including one or more activities, outputs and importantly expected outcomes that contribute to
the phasing out of animal testing in the regulatory assessment of chemicals. The catalogue will be a living document and made publicly available.
Notification of any initiative contributing directly or indirectly to the replacement or reduction of
animal use in the safety assessment of chemicals is welcomed. Notifications can be made by individuals on behalf of any stakeholder or team of stakeholders (e.g. academia, industry, EU agencies, Member State authorities, NGOs).
|
|
COCOPREND compiles and distills resources to facilitate responsible and ethical use, sharing and management of preclinical (animal) neuroimaging data. This data code of conduct has been developed by the
Center for Reproducible Science (CRS) of the University of Zurich. This website is meant to be a living document that is regularly edited and updated by the community.
COCOPREND includes relevant sections on such topics as reproducibility, Open Research Data (ORD), preregistration, the FAIR principles, Data Management Plans and legal and ethical considerations. The
PREPARE and
ARRIVE guidelines are both given space.
It can be
downloaded as an 80-page pdf file.
|
|
Spurred on by difficulties in obtaining barbiturates, Megumi Kiyoto and coworkers have evaluated
combinations of of medetomidine, midazolam or alfaxalone, and butorphanol as an alternative for euthanasia of mice.
Tilo Weber and colleagues have published an important paper on the use of
Fetal Bovine Serum (FBS) and how to leave it behind in the pursuit of more reliable science.
Ben Ineichen and colleagues at the University of Zurich, who are experts in literature analysis, describe their experiences in building a system for
information extraction from preclinical animal studies and the development of the PreCliniE model.
Submissions are invited for a special issue of the
Journal of Applied Animal Ethics Research on "Ethics and Welfare in Wildlife Research and Management", to be edited by
Miriam Zemanova. The deadline is
15 November.
Have you heard of the
Journal of Animal Law, Ethics and One Health? It was launched in 2023 by the Center for Animal Law and Ethics (CALE) at the Faculty of Law, University of Zurich, Switzerland. The main objective of the LEOH is to provide a forum for the discussion of legal and ethical issues relating to animals as well as the One Health approach and its implications for the law and the ethical realm. Furthermore, the Journal aims to provide a platform in which to explore
pivotal case law, legislation and political decisions that relate to or have an impact on animals. Norecopa has compiled an
overview of journals and other repositories, and tips on writing papers.
Amy Veness and coworkers have investigated the use of
customised combinations of environmental enrichment to reduce aggression in CD-1 male mice.
Sheffield researchers have developed
cell-based assays to replace mouse toxicity tests for detection of clostridium and tetanus toxins.
Daniël Lakens has written about
Improving Your Statistical Inferences. In addition, Norecopa has collections of papers by
Michael Festing and
other authors.
Cort Breuer and coworkers have published detailed
guidelines on the use of spontaneous and experimental models of lymph node metastasis.
Specific guidelines of this type are needed to supplement more general planning guidelines such as PREPARE. Indeed the authors state:
'Animal experiments to model metastases require careful planning to minimize the number of necessary animals and reduce variability. We encourage all practitioners to adhere to the PREPARE (Planning
Research and Experimental Procedures on Animals: Recommendations for Excellence), OBSERVE (Oncology Best-practices: Signs, Endpoints and Refinements for in Vivo Experiments) and ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines when planning, conducting and reporting animal experiments, except where otherwise noted'.
Norecopa has become aware of a textbook on
Teaching Fieldwork in Geography, Earth and Environmental Sciences. Although it is behind a paywall, the references listed in a chapter on
Ethics in fieldwork teaching suggest that this addresses animal projects, and should be of interest to those conducting field research.
Jennie Redander and colleagues describe the development of
a novel virtual reality simulation module for canine laparoscopic ovariectomy.
|
|
Stefano Gaburro has written a piece on LinkedIn describing
strain-specific responses to tunnel handling versus tail picking in mice. The comments section of his article are also worth reading.
Scientists in Finland have evaluated
the NOR test for mice (Novel Object Recognition task), which is a widely used memory test, and found concerns about its specificity and reproducibility.
In July the NIH reconfirmed
their attitudes towards human-focused research and announced that
all new notices of funding opportunities that relate to animal models must now also support methods that avoid animal use.
Rather belatedly, we can mention a paper from 2023 reporting what many will be intuitively familiar with: experimenter familiarisation is a crucial requisite for assessing behavioural outcomes and reduces stress in mice, not only under chronic pain conditions.
Neel Meyer and colleagues claim there is
lack of evidence for a consistent differential impact of tail and tunnel handling in welfare markers in laboratory mice. Contrary to what has been reported in literature, the results in this study show no consistent pattern indicating that tunnel handling is less stressful than tail handling. While some significant differences were observed across tests and analyses when comparing groups, all together they vary and do not show
a clear advantage of either method. Behavioural outcomes were mostly comparable between handling types and physiological markers of acute and chronic stress did not differ. A few handling-related effects were observed in C57BL/6J mice compared to CD-1 mice, but the findings were not consistent enough to conclude strain-specific sensitivities.
Lauren Hope and Jarrod Bailey have published a detailed account of their opinions on
how to break down barriers to animal-free research.
Rachel Heyard and colleagues have published a
scoping review on
metrics to quantify reproducibility - the second part of the title being
A multitude of questions leads to a multitude of metrics. The paper also includes a detailed account of
the differences between reproducibility and replicability.
The
VICT3R project was founded to improve reproducibility and reduce animal use in preclinical research. They invite scientists to
take a survey aiming to identify procedures in basic and translational research where historical data can be used to develop collaborative datsets and
Virtual Control Groups.
Megan Higgs and Valentin Amrhein take a fresh look at the subject of
power in statistical analysis.
And on a similar topic, Alexander Bird and colleagues make the point that
pilot studies should not be used to estimate sample size if the effect size and population variance are unknown.
Colby Vorland and coworkers have published advice on
the statistical analysis of grouped data in preclinical research: so-called clustering and nesting which occurs when animals are group-housed, or in cell culture.
Canadian scientist Hugh Townsend and his colleagues have published
a call to action to address critical flaws and bias in laboratory animal experiments and preclinical research. Focusing on experimental design in vaccination studies in high-impact journals, their findings contradict previous reports suggesting that rates of blinding and randomisation in laboratory animal studies are increasing. The discrepancy arises because previous studies focus on identifying any mention of randomisation
and/or blinding, whereas their focus was on the use of full implementation of both procedures.
Clare Stanford has written
an appraisal of preclinical investigations to predict the efficacy of psychedelic drugs as fast-acting depressants. She makes the point that each incident of drug failure increases the pressure to justify the use of animals. This is especially the case with drugs that have already been used extensively by humans. The paper discusses the reasons for these failures. She endorses Norecopa's
PREPARE guidelines as 'the first consideration' in a step-wise approach to the study design, with its 'broad checklist of factors that need to be addressed before embarking on any experiment that uses animals', as well as the
iTRIPP guidelines.
Springer Nature has collected the views of 11,000 scientists on their
attitudes to sharing null results.
An international team led by Terje Svingen at the National Food Institute in Denmark has examined the way
in which endocrine disruptors are identified in the EU, since they pose significant risks to human health and the environment. The EU prioritises their identification and is developing a roadmap to phase out animal testing while advancing New Approach Methodologies (NAMs), but the identification still heavily depends on
in vivo data until reliable alternatives are accepted and routinely applied. Efforts to reduce, refine, and replace animal testing are essential during this transition.
David Mayo at IDEXX BioAnalytics has
interviewed Professor emeritus Paul Flecknell about his 40-year long career as a laboratory animal veterinarian. Their collection of
Research Forward Podcasts is well worth examining.
In addition to the debate around gene editing and "de-extinction" of animal species, the question is now being asked
whether scientists should be allowed to bring distant human ancestors back to life? Arthur Caplan describes the limitations of cloning and CRISPR editing in both animals and hominids, and discusses the ethics and practical implications of de-extinction, if it indeed was possible.
And finally:
Can reviewers
recognise text generated by artificial intelligence?
P.S. no text in Norecopa's newsletters, or any of our text on the Norecopa website, has ever been generated by AI.
|
|
Dyrevernalliansens forskningsfond utlyser kr. 1.250.000 kroner til forskning og utvikling til beste for dyrene. Søknadsfristen er
26. september.
Fokus i år er dyrevelferdstiltak for kylling, verpehøns, gris og oppdrettslaks, men søknader om erstatning av forsøksdyr vil også bli vurdert.
Prosessen beskrevet høyere opp i nyhetsbrevet om endringer i EU-direktivet
er
beskrevet på norsk her.
|
|
Please help us in this task by forwarding this newsletter to friends and colleagues who may wish to subscribe. The white box at the bottom right of every page on Norecopa's website, or this link can be used.
|
|
|
Earlier editions of Norecopa's newsletter can be read here. They were published in Norwegian up to no. 2-2017. Free text searches on
Norecopa's website will also find resources which we have described in newsletters.
Mention of an institution, publication or professional service in these newsletters does not necessarily mean that Norecopa endorses all aspects of the activity. Norecopa and its staff are not involved, financially or otherwise, in these external activities unless this is explicitly stated.
|
|
Content:
Norecopa
Editor:
Adrian Smith
Org.no. 992 199 199
Bank account: 2801.53.03931
Vipps: 889149
All photographs in the newsletters have been taken by Norecopa or from
colourbox.com, unless otherwise specified.
You can read about
Norecopa's data protection and privacy policy here.
In compliance with the EU Data Protection Regulation (GDPR), Norecopa updated its personal data and privacy policy in 2018.
You can read about this here.
|
|
|