|
Newsletter no. 5-2025 from Norecopa
Welcome to Norecopa's fifth newsletter of 2025. This is the 130th newsletter which we have issued!
We hope you find them of use. We
welcome feedback, positive or negative.
Please share this newsletter with your colleagues and friends, and encourage them
to subscribe!
We are also on
LinkedIn and (to a much lesser extent) on
Facebook.
This newsletter contains the following items (if some links do not appear to work, check that your mail program has opened the whole of the newsletter):
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
|
Norecopa's 18th Birthday was on Friday 10 October. We
celebrated the occasion on Tuesday 14 October, in collaboration with the
National Committee for the Protection of Animals Used for Scientific Purposes and the
Finnish 3R Centre FIN3R, who both had scientific meetings that day.
The presentations from the day's events are all available online (all but the last one in English):
•
Norecopa 18 years: What have we achieved and what more should we aim for? (Adrian Smith, Norecopa)
• Experiences with the
Danish 3R-Center (Axel Kornerup Hansen) (pdf)
• A
seminar organised by the National Committee and including the following two presentations:
•
A presentation of the review paper: The welfare and ethics of research involving wild animals: A primer (Carl Soulsbury, University of Lincoln)
•
The 5Rs and best practice in wild terrestrial mammal research (Jon Martin Arnemo, University of Inland, Norway)
•
The role of ETPLAS, course modules, DOPs and SOPs (Nuno Henrik Franco)
• FIN3R webinar:
Supporting Replacement in Academia (Barney Reed, RSPCA) (Recording)
•
Refleksjoner over status og utfordringene knyttet til dagens dyreforsøk i Norge (Gunvor Knudsen, Mattilsynet)
We thank all those who have contributed to the establishment and work of Norecopa, from 2001 onwards when the first steps were taken to found what would become a national platform working to advance all the three Rs through
consensus between the four stakeholders: Regulators, Industry, Research and Animal Welfare.
Would you like to contribute to Norecopa's Board? Deputy Board Member Tone goes on maternity leave from the beginning of March. We are therefore looking for a substitute for her role, representing Industry.
|
|
The 4th Edition of
The IACUC Handbook was published in October. IACUCs (Institutional Animal Care & Use Committees) can, among other topics, read chapters on animals used in agricultural research and teaching; invertebrates; pandemic and disaster planning; operational communications and workflow; reduction of regulatory burden; and research reproducibility.
Stanford University have set up a visually very appealing website and
initiative entitled
Beyond3Rs, which aims to push the 3R concept further and 'draw on modern animal well-being science to pioneer a paradigm shift in the care and use of research animals'.
'Rats don't mind injections, it's the restraint they don't like'. These words are the start of
a video introducing
the 3Hs initiative by Emma Robinson at the University of Bristol - handling, housing and habituation.
The NC3Rs has released a report on
incorporation of New Approach Methodologies (NMAs) into medicines development.
The French 3R centre FC3R has compiled an excellent set of guidelines, in English, for good practice in the use of animals for scientific purposes,
BAFis. The collection includes guidance for animal models of specific pathologies: cardiovascular, infectious, neurological, oncological, renal and rheumatological.
The Centre also cites
Xemo, a
Xenopus model produced by a consortium of aquatic model experts to replace animal use in education and training. They also highlight
ExAVir, a French-language program for University students aiming to reduce the use of animals in practical work while ensuring an interactive and innovative teaching approach.
The Swedish 3R centre has recently published several sets of guidelines (only available in Swedish at present):
• a detailed
strategy for replacement, reduction and refinement of animal use
•
advice on non-aversive methods of administration of medicines and other substances
•
advice on gentle methods of handling, habituation and training of laboratory animals.
The UK Home Office commissioned the Animals in Science Committee to evaluate the quality of Non-Technical Summaries and Retrospective Assessment of animal procedures.
Their report is now out.
FELASA and EFAT have formed a Working Group
to develop learning outcomes for lab animal caretakers, technicians and technologists. The Group will develop outcomes for each level according to the report of the FELASA Working Group on
Harmonisation of education, training and continuing professional development for laboratory animal caretakers, technicians and technologists.
FELASA have also a
Working Group on communication in animal research, in collaboration with
AALAS,
CALAM,
CALAS and
EARA.
The Institute of Laboratory Animal Science at the University of Zurich have
published all their Standard Operating Procedures online.
Sheffield Post-doc Jo Sharpe has made a set of
Biological Research Trump Cards, a game designed
to help communicate 3Rs research for professional and public audiences.
SAALAS will host a workshop on Replacing the Use of Animals in Science in South Africa (planned for late 2026 in Gauteng) and
are seeking information from individuals and institutions in South Africa about the current challenges in transitioning to non-animal alternatives in your research facilities. The deadline for initial input was 12 November, but later input will no doubt be welcomed as well.
Are you comfortable about the way that AI will impact your research? The 3Rs Collaborative has
launched
a webinar series on the subject (a recording of the first one
is available here).
A recording is now available of a webinar by Ivo Tiebosch on the
EU module 27 for training competence assessors.
|
|
The website of the European network of 3R centres, EU3Rnet, is now up and running. Colleagues are invited to link to this site, and to keep EU3Rnet updated on news and events.
Comments and suggestions for additions to the website are welcome.
|
|
The NATworks platform offers an overview of companies, products and services providing Non-Animal Technologies for human-relevant research. Items can be searched by filters including country, endpoints, organ systems and research fields.
|
|
Former Norecopa Board Chairman Chris Noble is lead author of a 85-page Open Access
review of welfare indicators for Atlantic salmon (Salmo salar), rainbow trout (Oncorhynchus mykiss), European seabass (Dicentrarchus labrax), gilthead seabream (Sparus aurata), and common carp (Cyprinus carpio). This review was part of the EU-funded
AquaExcel 3.0 project.
Norecopa's secretary is among the over 50 co-authors. He is also co-author of three other manuscripts which are under review in other journals. More information on these will be given i future newsletters.
The first webinar of the
Nordic Network on Fish Welfare (NNFW), entitled
Fish Welfare in Fisheries, will be held on 3 December.
Fish count, too: Jean Knight and colleagues document the animal toll of REACH aquatic toxicity tests.
|
|
Christopher George and colleagues encourage scientists to be
CLEAR (Clarity, Evaluation, Assessment, Rigour) to ensure methodological and data transparency.
Charles River have published their programme for
early rabbit to human habituation to reduce stress and aggressive behaviour.
NestLab devices are winners of the
NC3Rs CRACK IT challenge, developed to provide thermal control and continuous health monitoring for mice and rats. Better home cage monitoring
aims to refine and reduce animal use in drug safety testing.
Why catch when you can throw? Inspired by plant burs, Rory Wilson and coworkers have developed a novel method of non-invasive marking of wild animals. The paper contains a large number of references which will be of interest to field
researchers.
Tomasso Virgilio and coworkers have examined
the impact of several anaesthetic combinations on vital parameters and immune responses in mice.
Sebastian Brachs and coworkers have developed
a novel urination marker to enable robust, non-invasive detection of hyperglycaemia in mice.
Janine Feige-Diller and coworkers have investigated
the effects of different feeding routines on the welfare of mice.
Lydia Smith and Amy Orsborn have developed a customizable
head simulator for use in planning and training personnel within neurophysiology and neurosurgery in non-human primates.
Suguru Yamauchi and coworkers have described
a new method of external jugular venipuncture in mice, which they state improves the precision and reproducibility of the technique.
Sander Prins and colleagues have investigated
stress and behavioural responses in sheep when used in teaching for veterinary undergraduates.
A new COST Action has just started,
CA24168
to enhance the current Sex as a Biological Variable (SABV) initiative. This should extend to
in vitro research as well as
in vivo studies.
The EU's Joint Research Centre has announced
a review of models and methods used in cardiovascular research.
Frontiers in Technology has a series of papers on cell culture, with
a focus on how to avoid using animal material.
Maria Karlgren's research group have demonstrated that
3D primary hepatocyte spheroids can be grown in cultures where foetal bovine serum (FBS) has been totally replaced.
Helena Hogberg and Lena Smirnova have written a review about
the future of 3D brain cultures, in particular in relation to developmental neurotoxicity testing.
The US National Institutes of Health have created a national hub for human
in vitro methods: the
Standardized Organoid Modeling (SOM) Center. The centre's aim is to serve as a neutral scientific hub, developing organoids that are reproducible, reliable, and easily accessible for medicinal and biological research.
The
ECA Foundation's
Academy has
commented upon the fact that the NIH will no longer award funding for new grant applications that rely exclusively on animal testing, and that the WHO is following suit with new guidelines entitled ‘Guidelines on the replacement or removal of animal tests for the quality control of
biological products’.
Anna Niarakis and colleagues describe how the development of
Digital Twins within immunology can benefit personalised healthcare.
Julia Bugajska and colleagues discuss
how long it takes to complete and publish a systematic review of animal studies.
|
|
Hugh Townsend and colleagues call for
action to address critical flaws and bias in lab animal experiments and preclinical research. Among other things, they report that as few as 0–2.5% of a random sample of comparative laboratory animal experiments conducted in North America and Europe and published in 2022 used valid, unbiased experimental design. A primary objective of the paper is to help biomedical scientists understand that a necessary requirement for
preventing bias in laboratory animal experiments involves a combination of controlling for cage effects, implementing full investigator blinding, and fully randomising study procedures.
There is an ongoing discussion about these findings and their cause,
in the form of a blog with comments from other specialists, which is also highly recommended.
University of Washington Professor Sally Thompson-Iritani has written a paper applying a SWOT analysis to critically evaluate the current landscape of animal research:
Animal Research at a Crossroads: Strengths, Weaknesses, Opportunities, and Emerging Threats.
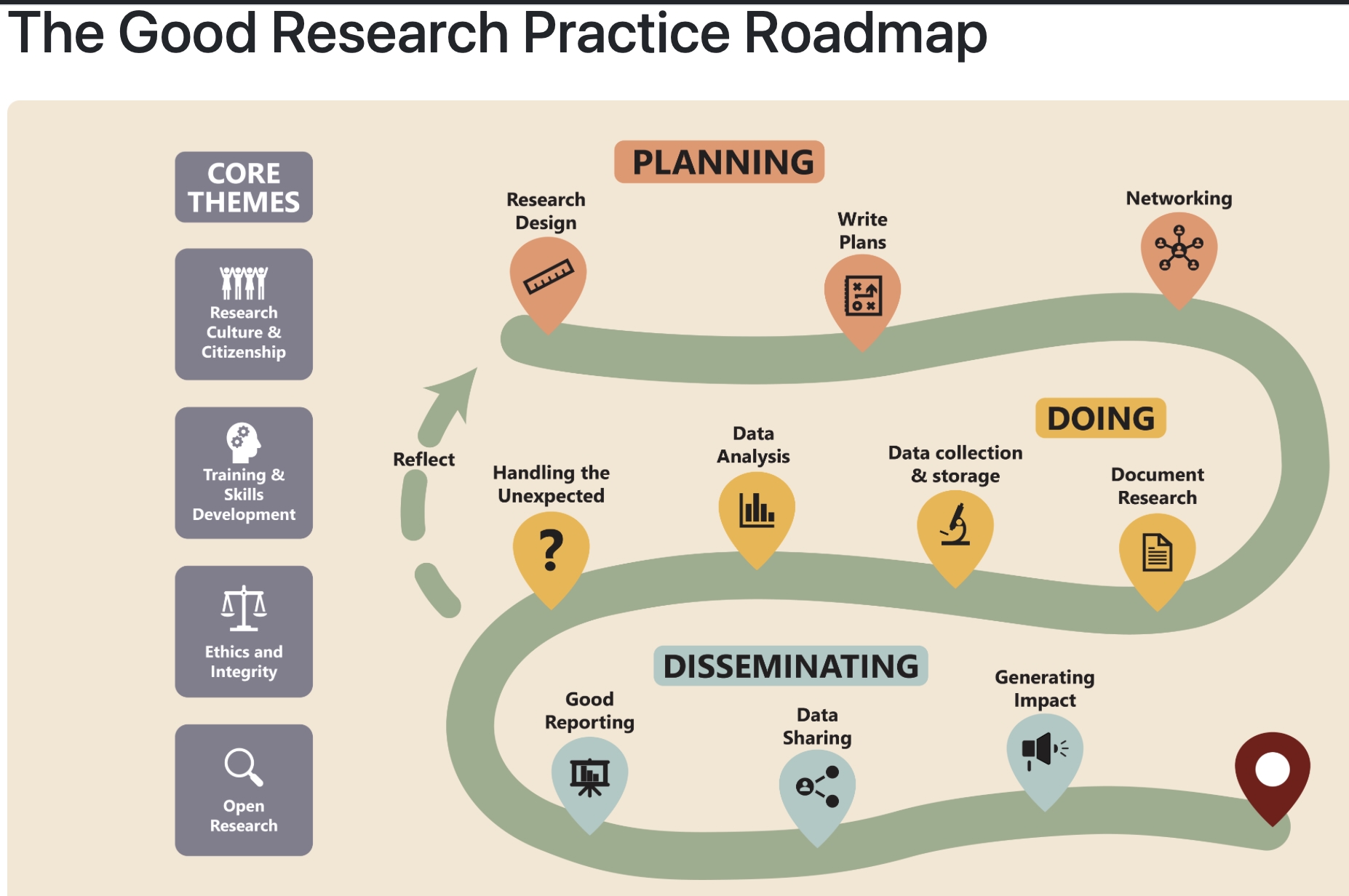
Gillian Currie and coworkers at Edinburgh University have produced a
Roadmap for Good Research Practice (see illustration), detailing the importance of all the steps in planning, conducting and disseminating research, ensuring it is transparent, of high quality, ethical, and impactful.
The production of Non-Technical Summaries and Retrospective Assessment of animal studies are key elements of European legislation. The UK Animals in Science Committee has
published a review and recommendations about their use. Their conclusions are relevant to other countries too.
The NC3Rs has published a report highlighting the impacts of the first UK funding call for
infrastructure to strengthen national capabilities in methods that replace the use of animals. Eleven awards totalling £3.95M were made through a competitive call. The funding enabled national facilities to be established for stem cells, bioprinting, organoids and organ-on-a-chip systems, providing greater access to these technologies as well as training opportunities for
researchers.
Katharina Ameli and Stephanie Krämer have conducted a survey to evaluate
the implementation of Culture of Care in Germany. The results indicate that it has not been fully established. Individual institutional steps for implementation are proposed.
The Home Office has published its
statistics for the use of animals in Great Britain in 2024. Understanding Animal Research have commented upon these, pointing out that
ten organisations account for a third of all the animals.
The RSPCA
has also commented that this represents a decrease of 2% from 2023, and the lowest figure since 2001. They also welcome a further decrease in the number of experimental procedures causing
‘severe’ suffering, an overall decrease since 2014 of 68%. Regulatory testing accounted for 35,511 of the experimental procedures causing ‘severe’ suffering, with batch potency testing responsible for over 57% of these. The RSPCA are calling for more transparency about what happens to animals who are bred for use in research, but not used in scientific procedures (for example, because they are killed for studies that require their tissues,
or do not have a required genetic alteration). Omitting this important information may hinder efforts to successfully implement an effective phase-out. The European Union collects and publishes these ‘bred, not used’ lab animal statistics every five years, but the last time the UK published this kind of data was for the year 2017. These data are needed to fully understand and measure the true impact of science on animals.
Emma Roe and colleagues have worked on creative pedagogic methods
to see how curiosity and creativity can be harnessed to engage people with different views on animal research.
The Swiss 3RCC has published infographics summarising the results of two surveys they conducted on
the use of non-aversive handling methods.
What barriers are there to implementation of the R of Replacement? An NC3Rs
submission to a Government request for opinions on
Technology alternatives to animals in life sciences research may be of interest.
Despite growing evidence for the value of pre-registration of study design and analysis plans, its use is still uncommon in preclinical research. Cristina Priboi, Hanno Würbel and colleagues have conducted
a survey to assess the reasons for this.
Veterinarian Tanya Stephens and colleagues have published a book on
veterinary controversies and ethical dilemmas, with 'provocative reflections on clinical practice'.
David Shaw and colleagues conclude that the 3R principles and the concept of Harm-Benefit Assessment
rule out any homeopathy research on animals.
Jessica Rosolowski, Tilo Weber and Atena Malakpour-Permlid have
revisited the 3Rs, rethinking Replacement and New Approach Methodologies.
Judith Homberg and colleagues describe
methods to improve communication about animal and animal-free research methods.
Stefano Gaburro has written
a blog on how to unlock clinical progress, with emphasis on digitalisation,
FAIR data and collaborative transparency.
The US Accountability Office has published their
assessment of Human Organs-on-A-Chip, concluding that technologies offer benefits over animal testing but challenges limit wider adoption.
The Dutch funder ZonMW has introduced an
Animal-Wise Policy, encouraging researchers to do their research without animal testing - and, if animal testing is necessary, that this is performed as well and transparently as possible. They ask applicants, referees and committee members to take a critical look at the necessity and implementation of the animal experiments, and they set specific conditions for successful applications that increase the quality and transparency of animal
testing and can prevent unnecessary repetition.
Pandora Pound has written a critique of animal research, approaching the challenges of replacing animal research i academia from a sociological perspective, and
describing the contribution of Bourdieu.
Anis Barmada argues that
modern animal models remain far better than alternatives.
Andrew Knight has published a collection of 25 videos on
the use of animals in education and training, and their alternatives. The collection also contains videos on the usefulness of animal research - a starting-point for discussion, regardless of your views on the subject.
EARA comment upon
two articles with opposite perspectives on animal research in
Nature.
Thomas Hartung has written a review of the increasing use of AI and automation in research, which he calls
the beginning of scAInce.
|
|
Norecopa deltok i den årlige høringen hos Næringskomitéen om regjeringens forslag til neste års budsjett (innlegget,
høringsnotatet og
opptaket).
Une Bastholm (MDG) sendte et skriftlig spørsmål til fiskeri- og havministeren om deler av FoU-avgiften som innkreves fra sjømatnæringen kunne brukes til å finansiere forskning på 3R-alternativer, inkludert drift av et nasjonalt 3R-senter. Ministerens
svar kan leses her.
På oppdrag fra den nasjonal forsøksdyrkomitéen har Sveriges 3R-senter laget
en detaljert strategi for å erstatte, redusere og forbedre bruken av dyr i vitenskapelige formål.
Bakgrunnen for endringene i EU-direktivet omtalt tidligere i dette nyhetsbrevet
kan leses her.
Forskning.no har intervjuet flere forskere og Norecopas sekretær om innsatsen på 3R-området, og
konklusjonen er at Norge henger etter.
Regjeringen har igjen i år
delt ut milliardbeløp fra Havbruksfondet til kystkommunene og fylkeskommunene som har fiskeoppdrett. 'Kommunene står fritt til selv å vurdere hvordan pengene best bidrar til å skape velferd for sine innbyggere', sier fiskeri- og havministeren. Norecopa har foreslått at et lite beløp fra fondet holdes tilbake for å finansiere et 3R-senter som kan gagne forsøksfiskene som oppdrettet er bygget
på.
|
|
Please help us in this task by forwarding this newsletter to friends and colleagues who may wish to subscribe. The white box at the bottom right of every page on Norecopa's website, or this link can be used.
|
|
|
Earlier editions of Norecopa's newsletter can be read here. They were published in Norwegian up to no. 2-2017. Free text searches on
Norecopa's website will also find resources which we have described in newsletters.
Mention of an institution, publication or professional service in these newsletters does not necessarily mean that Norecopa endorses all aspects of the activity. Norecopa and its staff are not involved, financially or otherwise, in these external activities unless this is explicitly stated.
|
|
Content:
Norecopa
Editor:
Adrian Smith
Org.no. 992 199 199
Bank account: 2801.53.03931
Vipps: 889149
All photographs in the newsletters have been taken by Norecopa or from
colourbox.com, unless otherwise specified.
You can read about
Norecopa's data protection and privacy policy here.
In compliance with the EU Data Protection Regulation (GDPR), Norecopa updated its personal data and privacy policy in 2018.
You can read about this here.
|
|
|