|
Newsletter no. 5-2024 from Norecopa
Welcome to Norecopa's fifth newsletter of 2024.
This is the
123rd Newsletter published by Norecopa since 2008.
We hope you find them of use! We
welcome feedback, positive or negative.
Please share this newsletter with your colleagues and friends, and encourage them
to subscribe!
We are also on
Facebook,
Twitter/X and
LinkedIn.
This newsletter contains the following items (if some links do not work, check that your mail program has opened the whole of the newsletter):
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
|
This summer has been an extremely busy one for Norecopa and others, reflected in the length of this newsletter.
A major upgrade of the Norecopa website was completed in August, thanks to generous sponsorship from Novo Nordisk, Scanbur, Aivero and Thorgate. Some of these improvements are visible (a new font, upgraded logo and icons, a new header on each webpage and improved newsletter design) but most are related to the way in which the 10,200 webpages are edited, displayed and stored.
This work was achieved without down-time, thanks to Norecopa's website clone which is used for testing new functionalities. We have also installed a better system for automatic detection of broken links on the site. Comments and further suggestions are always welcome.
The
PREPARE checklist has recently been translated into Albanian by Edlira Aruci, bringing the total number of languages up to 36. Of the EU languages, we still need Maltese and Lithuanian - would you like to undertake this? The PREPARE paper has now passed 37,000 views/downloads and is the
4th most read paper in its journal the last 6 months.
This year's
EUSAAT congress was held in Linz, Austria on 18-20 August. The day before, the
COST Action IMPROVE, in which Norecopa participates, held
a workshop and networking meeting. Adrian Smith participated in both these events.
At EUSAAT he served on the Scientific Committee, had
a poster about Norecopa, oral presentations on a
Culture of Care and
quality assurance in animal research, and a mentoring session for young scientists on career development. In addition, as Board Member he was on the jury for two Young Scientist Travel Awards of 250 euros each from
ecopa, based on the abstracts of their oral presentations at the congress (photo).
The
Congress programme featured many high-level presentations on aspects of all three Rs (abstract book).
One of the sessions was on funding 3R research. The Dutch funder ZonMw has investigated possible ways of reducing the bureaucratic burden on researchers. Their report,
Stimulating Transparent Laboratory Animal Research, also offers insights into methods that can increase the transparency of animal research, which should lead to more efficient and better animal research and a reduction in unnecessary repetition.
The next Congress, which is highly recommended, especially for early career scientists, will probably also be in Linz in 2026.
|
|
Norecopa's
Summer School on Systematic Reviews and Literature Searching, in Bergen 20-23 August, was a great success, scoring 64 of 65 possible points on the students' evaluation forms. The students used a research question taken from their own project to learn the various steps involved in conducting a review, and worked hard to make the most of the tutors from the STRIDE Lab in Zurich who were present. Norecopa treated them one afternoon to a trip up the
funicular to the hills above the city to give them a break from the studies, as well as a "pub quiz" one evening.
We have provisionally booked the same venue for a repeat of this School on
19-22 August 2025, while we look for funding. Those who might be interested are welcome to contact
Adrian Smith.
|
|
As mentioned in our previous newsletter, Adrian Smith attended two very successful meetings in Utrecht in June.
The first was a meeting of the COST Action
IMPROVE.
The other meeting was a conference entitled
The 3Rs and NAMs: all-inclusive? organised by the Utrecht 3Rs Centre. A
report from the meeting is now available, along with
a photo gallery. The report's title includes "respectful dialogue", a phrase contributed by Adrian Smith at the start of the meeting when the participants generated a word cloud to visualise their intentions with the meeting. He had a
poster about Norecopa's work at the meeting.
The Centre has made a short video film about
Connecting the 3Rs and NAMs.
An
introduction to the IMPROVE project has recently been published in ATLA.
Following these events, Adrian Smith has published some thoughts about
the use of the acronyms NAMs and NATS, both of which are being used, sometimes with different meanings, in the debate about phasing out animal research and testing. This webpage will be expanded as new insights and papers appear.
|
|
John Parascandola, a medical historian at the University of Maryland, has just published
A History of the Development of Alternatives to Animals in Research and Testing. This important book traces the development of the concept of alternatives up until Russell & Burch published their book about the 3Rs in 1959, and (not least) fills a gap by describing in detail the events
after its publication, including a decade of lack of interest in the 1960s, until alternatives "came of age" in the 1980s. Parascandola has interviewed several of those who knew Russell & Burch well.
Many papers have been written, and opinions voiced, about the 3R principle (links to many of them are available here). Many have not read the original version of
The Principles of Humane Experimental Technique from 1959, nor the reprint in 1992, which
are both difficult to obtain. An abridged version by Michael Balls, written in simpler English to celebrate the 50th anniversary of the book, can however
be downloaded free of charge from FRAME.
In this connection, Nuno Franco's paper giving
a historical perspective to animal experiments in biomedical research is of interest. Links to more papers may be found on Norecopa's webpages on
ethics and on
the 3Rs.
|
|
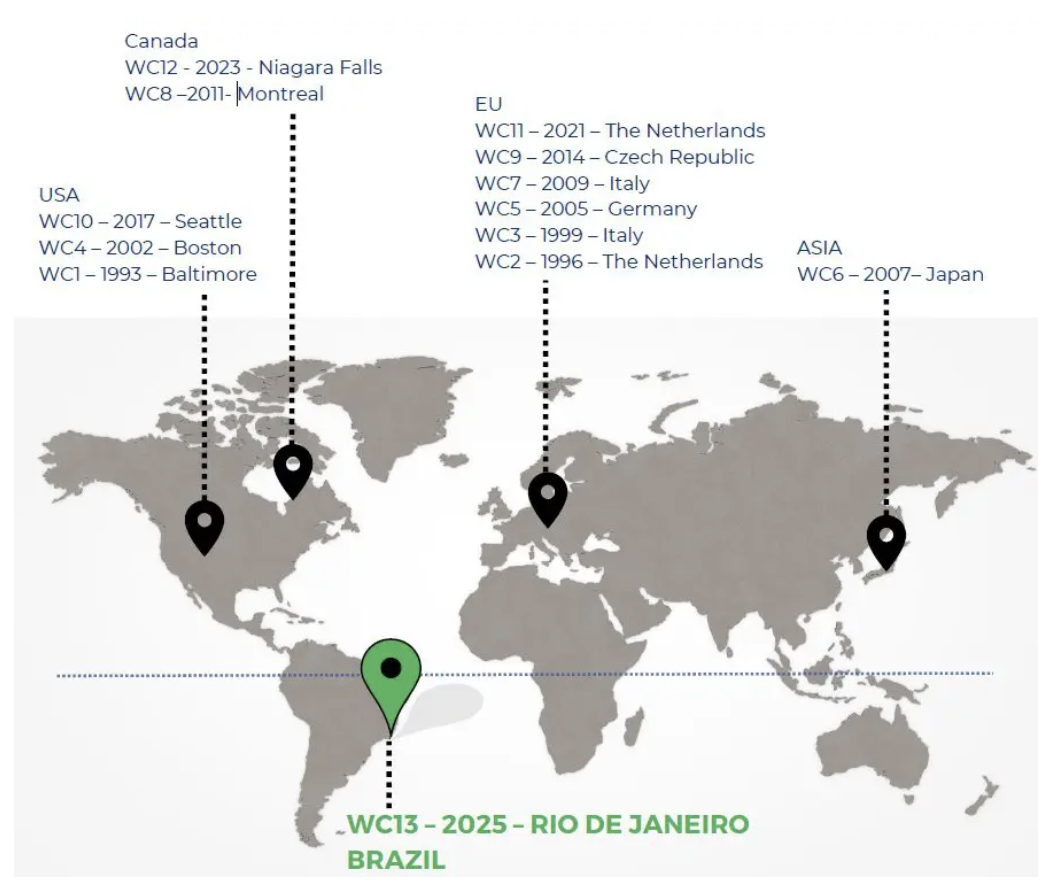
Norecopa's Secretary is serving on the
Scientific Committee of the next
World Congress on Alternatives, which will be the 13th Congress, to be held in Rio de Janeiro in August next year. These congresses, which have been held since 1993, focus on all three Rs, although the R of Replacement, particularly within regulatory toxicology, has traditionally occupied most space. Session proposals can be
submitted until 15 November.
The deadline for submitting proposals to organize the next (14th) World Congress on Alternatives and the Use of Animals in Life Sciences has been extended to November 1st, 2024. We encourage all interested parties to take advantage of this additional time to submit their applications. More information is
available here or from Adrian Smith.
|
|
The statistical data on animal use in the EU for 2021 and 2022 were published this summer in the
ALURES database. The total number of animals used in 2022 in the was just under 7 million. The use of animals in regulatory testing has decreased by around half a million animals compared to 2018. The number of rats and mice has decreased by around 10% decrease over the last five years. The top three EU countries for animal use in 2022 were France (1,8 million animals), Germany (1.3 million) and Spain
(1.05 million). In 2022, 90% of the total number of animals were mice, fish, rats and birds. Dogs, cats and monkeys account for around 0.23% of the total (EARA infographic).
Starting with the data from 2021, it is now possible to access data at the Member State level. Norway's statistics are included.
New e-modules for the functions defined by EU Directive 2010/63 are under development and
ready for testing: please participate.
A working group appointed by ETPLAS has issued
guidance on the assessment of learning outcomes for those designing procedures and projects. ETPLAS has compiled
a list of European courses in Laboratory Animal Science, and hosts free
EU-Function modules (learning resources tailored to the functions defined in the EU Directive 2010/63). Links to more international courses are available on
Norecopa's website. Additions or amendments are always welcome.
The main findings from the European Commission workshop on “The Roadmap Towards Phasing Out Animal Testing for Chemical Safety Assessments” (held in Brussels in December 2023) have been published. The roadmap is intended to be a policy
document which will outline milestones and specific actions, addressing all relevant pieces of chemical legislation relating to Safety Assessment. The roadmap intends to analyse and to describe the necessary steps to replace animal testing in pieces of legislation that currently require animal testing for chemical safety assessments. The roadmap will outline the path to expand and accelerate the development, validation and implementation of non-animal methods as well as means to facilitate
their uptake across legislations. The roadmap is planned to be finalised in the first quarter of the term of the next Commission i.e. end of 2025 / beginning of 2026.
The EU Commission
PRO-MaP initiative (recommendations to improve methodological clarity in life sciences publications) cites Norecopa's
PREPARE guidelines as one of six recommended resources for researchers.
|
|
The Commission of the European Pharmacopoeia has adopted revised texts which
will completely remove the Rabbit Pyrogen Test (RPT) with effect from 1 July 2025. Parenteral medicines are tested for their pyrogenic effect, and the RPT (where rabbits are injected intravenously with the substance and their body temperature recorded) has been the standard for decades. The RPT has been
criticised for many years.
In vitro tests such as the
monocyte-activation test (MAT) will take its place (this link is to a commercial company, but it gives a good overview of the MAT and its alternatives).
|
|
Bernhard Voelkl and Hanno Würbel discuss
heterogeneity of animal experiments and how to deal with it.
Organs-on-chips are not generally associated with cancer research, but Elisa Cauli and coworkers describe their use
to study the microenvironment of a tumour.
Those wanting an overview of the status regarding organs-on-a-chip may find the websites of the
International MPS Society and the
3Rs Collaborative helpful. In July, an
International Roadmap for Standardization of Organs-on-chips was launched at the EUROoCS 2024 meeting in Milan. The Roadmap
can be downloaded here.
Cancer research can now use the
OBSERVE guidelines for the refinement of rodent cancer models.
Ilya Smolensky and coworkers have examined
the effects of single housing on behaviour, corticosterone levels and body weight in male and female mice.
Are camera traps and collection of environmental DNA equally effective? Eleanor Di Girolamo and coworkers
have investigated this in connection with mink in Indiana.
Juliet Lamb and coworkers demonstrate that
implanted satellite transmitters affect sea duck movement patterns, both in the short and long term.
The
Laboratory Animal Genetic Reporting framework (LAG-R) has been developed to help scientists publish a more comprehensive description of the genetics of research animals, to improve data interpretation and reproducibility.
Fernando Benavides and Axel Kornerup Hansen have edited a book entitled Rodent Quality Control: Genes and Bugs. It includes chapters on
health and genetic monitoring of laboratory animals, and
laboratory animal pathology in relation to spontaneous infections.
Julia Swan and colleagues demonstrate
how refinement (In this case gentle handling) affects drug endpoints and stress responses in mice. Likewise, Canadian scientists (Marcotte
et al.) have published a protocol on
protocols.io which focuses on habituating mice to their handlers over a 3 day period.
Katharina Hohlbaum and colleagues have designed
mechanical puzzles ("lockboxes") for use in mouse cages to allow them to express more of their behavioural repertoire. The lockboxes may also be used as a novel approach for assessing cognition in mice. The work is published in
Open Research Europe, an open access publishing venue for European Commission-funded researchers across all disciplines, with no author fees, open peer review and indexing in databases such as PubMed.
Good Practice guidelines for preclinical alcohol research (STRINGENCY) have now been published.
Michelle Gygax and coworkers
compared enriched and conventional mouse cages, demonstrating the significance of a proper substrate for shelter, and other resources that facilitate species-specific behaviour. Similarly, Oddrun Gudbrandsen has demonstrated that
periodic stays in a "playcage" function as environmental enrichment for rats that are housed in individually ventilated cages.
Daniel Lukovikov and colleagues have published a novel
open-access AI-driven platform for CNS drug discovery using adult zebrafish.
The journal
Animal Welfare can now be
accessed via LinkedIn.
A paper by Margarita Bisio and colleagues in connection with
models of
Trypanosoma infection demonstrates the value of systematic reviews and meta-analysis.
Tim Whitney and coworkers are using
a chicken egg model in cancer research, utilising the blood vessels in the membrane surrounding the embryo.
The Jackson Laboratory has published
strategies to minimise genetic drift and maximise experimental reproducibility in mouse research.
Are you aware of the series of nearly 70
Statistics Notes by Doug Altman and Martin Bland? These cover a large range of subjects including topics such as one- and two-sided tests, presentation of numerical data and transformations.
Natasha Karp and colleagues describe
five common pitfalls in preclinical pilot studies and how to avoid them.
|
|
Judith Madden and colleagues have written a practical paper about
the publishing process and how to maximise research impact. They recommend the
PREPARE guidelines as part of the toolbox to make most use of the information obtained from an experiment.
Randy Nelson and coworkers argue that
more explicit consideration of the time of day when experiments are performed will improve experimental rigor and reproducibility.
The Lush Prize has written
a report about animal use in research. testing and education in Africa, based upon the fact that only one of 156 winning projects come from there.
David Colquhoun has written a review paper entitled
An investigation of the false discovery rate and the misinterpretation of
p-values.
Mike Dennis and coworkers offer advice on
facility design and management: in particular strategies for high-level containment.
Do we value negative equity? The value of reporting negative results is discussed by Owen Sansom and coworkers in a recent paper.
Making data and code easily available (both for ourselves and others) is valuable, as Samuel Gersham points out.
Annamaria Carusi has just published a paper entitled
Chemicals regulation and non-animal methods: displacing the gold standard, where she discusses the challenges in effecting a radical change in toxicity testing, particularly in the regulation of industrial and consumer chemicals.
The Mouse Metabolic Phenotyping Center (MMPC)
Live Program was established last year to characterise mouse models of diabetes and obesity. Their mission is to “advance medical and biological research by providing the scientific community with standardized, high quality metabolic and physiologic phenotyping services for mouse models of diabetes, diabetic complications, obesity and related disorders", The
PREPARE guidelines are cited in that connection.
Andrew Harrell and coworkers discuss the
endeavours made by trade associations, pharmaceutical companies and regulators to replace, reduce and refine animal use in safety testing.
The
COLAAB initiative has produced
a guide to avoiding animal methods bias in publishing.
Katy Taylor, Stephanie Modi and Jarrod Bailey have analysed
trends in the use of animal and non-animal methods in biomedical research and toxicology publications, concluding that, relatively speaking, the reliance on animals in these research areas is decreasing.
The ongoing debate about today's interpretation of the 3R tenet continues. Edwin Louis-Maerten and colleagues present
conceptual foundations for a clarification of their meaning. The authors have also written about
the ethics of xenotransplantation.
Mikalah Singer and Aysha Akhtar discuss
with what we should replace non-human animals in biomedical research protocols.
Rebecca Critser
asks how the 3Rs should be revised and why. She explores the merits and drawbacks of possible updates to and interpretations of the 3 Rs.
Pierfrancesco Biasetti and colleagues
discuss the biobanking procedures used in wildlife research and conservation.
How much 'enrichment' is enough? Jessica Cait and coworkers
address this question.
Scienxe has published
an issue with several feature article on rats - both in the laboratory and in their role as pests.
A selection of journals are soliciting papers to
a cross-journal collection highlighting aims to promote research that considers SABV (Sex as a biological variable).
Judith Homberg and colleagues discuss how we can
optimise communication about animal research and animal-free methods.
The veterinary workplace carries a high risk of staff accidents and has not been studied as extensively as in human medicine. Tamzin Furtado and colleagues have written about the
pain, inconvenience and blame.
Wen Tsin Poh and Johnson Stanslas have written
a review of the acceptance of the 3R principle in regulatory testing.
Christian Rodriguez Perez and coworkers
examine the 3Rs from an experimental bioethics approach.
Finally, Nico Müller asks:
why not just phase out animal experimentation?
|
|
Det svenske 3R-senteret har laget plakater (affischer) som sammenfatter
tips om akklimering av mus og rotter før forsøk. Disse og en rekke andre publikasjoner er også tilgjengelige
på engelsk.
Dyrevernalliansen har publisert en rapport med tittelen
Fremtidens fiskevelferd - med undertittelen 'tiltak og løsninger for bedre fiskevelferd, redusert dødelighet og økt bærekraft'.
Adrian Smith deltok på to av dagene under
Arendalsuka i august, hvor han bl.a. snakket med to statsråder, direktøren for Forskningsrådet og en rekke forskere.
Vi minner norske lesere om at man kan donere til Norecopa ved å bruke
Vipps, nr.
889149, med et valgfritt beløp.
På forhånd takk for støtten!
|
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
|
Please help us in this task by forwarding this newsletter to friends and colleagues who may wish to subscribe. The white box at the bottom right of every page on Norecopa's website, or this link can be used.
|
|
|
Earlier editions of Norecopa's newsletter can be read here. They were published in Norwegian up to no. 2-2017. Free text searches on
Norecopa's website will also find resources which we have described in newsletters.
Mention of an institution, publication or professional service in these newsletters does not necessarily mean that Norecopa endorses all aspects of the activity. Norecopa and its staff are not involved, financially or otherwise, in these external activities unless this is explicitly stated.
|
|
Content:
Norecopa
Editor:
Adrian Smith
Org.no. 992 199 199
Bank account: 2801.53.03931
Vipps: 889149
All photographs in the newsletters have been taken by Norecopa or from
colourbox.com, unless otherwise specified.
You can read about
Norecopa's data protection and privacy policy here.
In compliance with the EU Data Protection Regulation (GDPR), Norecopa updated its personal data and privacy policy in 2018.
You can read about this here.
|
|
|