|
Newsletter no. 1-2025 from Norecopa
Welcome to Norecopa's first newsletter in 2025. This is the 126th newsletter which we have issued.
We hope you find them of use! We
welcome feedback, positive or negative.
Please share this newsletter with your colleagues and friends, and encourage them
to subscribe!
We are also on
LinkedIn and (to a much lesser extent) on
Facebook and
X.
This newsletter contains the following items (if some links do not work, check that your mail program has opened the whole of the newsletter):
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
|
Norecopa has started to construct a webpage that gives
a global overview of funding sources for 3R projects. We
welcome suggestions for additions to the page.
Since the last newsletter, Adrian Smith has held a presentation for the Standing Committee on Business & Industry, in connection with the the Norwegian Ministry of Agriculture and Food's Report on Animal Welfare
(Dyrevelferdsmeldingen), and at a political seminar organised by the Norwegian Animal Protection Alliance. Both these were in Norwegian and are described in more detail in
the section for Norwegian-speaking readers at the bottom of this newsletter.
There is an article about the
PREPARE guidelines in
the latest newsletter from ANZCCART (Australian and New Zealand Council for the Care of Animals in Research and Teaching).
Two recent additions to Norecopa's
Refinement Wiki include a video introducing the
MDA technique (Micropipette-guided Drug Administration) for voluntary oral uptake by rodents, and videos of
Lockbox enrichment, a technique which has been shown in a variety of species to increase animals' manipulative and cognitive activities.
Do you have text, photographic material or experiences which could be published on one of
the 70 topics already in the Wiki, or on a new page? If so, please
contact Adrian Smith.
Likewise, we have recently added more material to
the resource website of the
International Culture of Care Network. Finland became a member this month - an overview
can be seen here.
The international accreditation organisation AAALAC has updated its list of "Reference Resources" for facilities conducting animal research and testing, accompanied by
a 4-minute video. The Resources list includes
Guidance on the severity classification of scientific procedures involving fish, produced by a Norecopa working group. The PREPARE guidelines are cited as a
general resource for investigators and as a source of
information on alternatives. AAALAC also maintains a useful
overview of regulations and resources by country, the presentations from their
2018 conference with ESLAV, ECLAM and SECAL, details of their
October 2025 conference and an
invitation to submit abstracts.
Norecopa is a member of AAALAC, following the positive experience with the organisation gained from accrediting the Laboratory Animal Unit at the Norwegian School of Veterinary Science in Oslo.
|
|
Norecopa's website now consists of over 10,000 pages of global resources to advance implementation of the 3Rs. Adrian Smith held a webinar recently where he gave a guided tour of the website, pointing out features which many are probably unaware of.
The webinar was recorded and
is available here on Norecopa's
Vimeo channel, which also contains many other videos that may be of interest.
Many thanks to Vootele Voikar of the Finnish 3R centre,
FIN3R, for kindly hosting the webinar.
|
|
In the UK, the Non-Technical Summaries (NTS) of all projects that have been approved by the Home Office
are made publicly available. The
template for these summaries includes questions such as
'What published best practice guidance will you follow to ensure experiments are conducted in the most refined way?' and
'What steps did you take during the experimental design phase to reduce the number of animals being used in this project?'
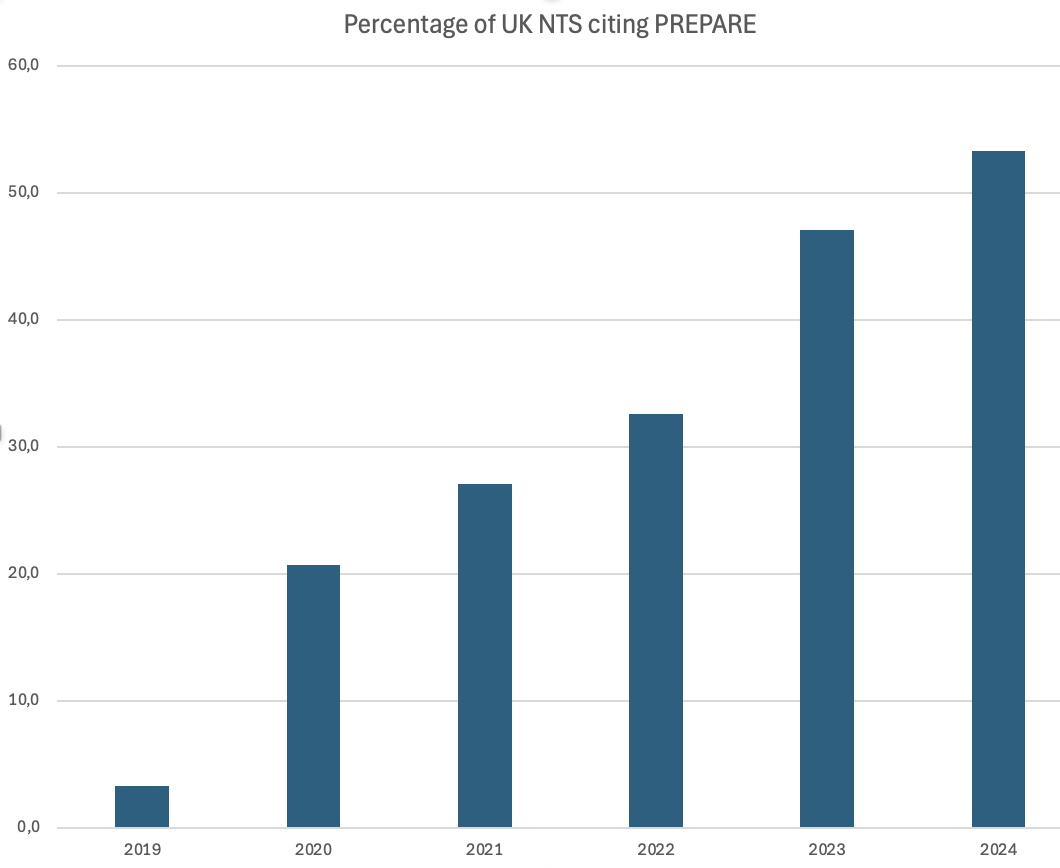
Norecopa has analysed all the 2,412 NTS published in 2019-2024 to examine the uptake of
the PREPARE guidelines for planning animal studies. A total of 729 projects cited PREPARE as one of the resources they used, and the percentage of NTS that do so has increased each year (see the figure), to just over 50% in 2024.
The UK Home Office recognises that Non-Technical Summaries (NTS) and Retrospective Assessments (RAs) are key for transparency on the use of animals in science to support the 3Rs.
It has commissioned the Animals in Science Committee for advice on standards for NTS and RA content that will improve openness and transparency and support the implementation of the 3Rs more effectively.
A review giving an
Introduction to conducting responsible and reproducible agricultural research is one of the latest examples of papers
endorsing PREPARE.
|
|
All those involved in education and training in Europe should consider participating in the conference
Shaping the Future of LAS Education and Training organised by ETPLAS in Leiden on 18-19 March. This is a golden opportunity to learn about the range of new resources now available for compliance with the EU directive.
The RSPCA have published three reports recently, from:
• their
"Focus on Severe Suffering" event held in collaboration with Newcastle University in November last year. The meeting featured two sessions: “Refining Pain in Pain Research”, focusing on reducing pain in studies where pain is the subject of research, and “Refining Pain in Painful Procedures”, addressing pain reduction in areas causing severe suffering without pain as the
objective.
• their
6th International Focus on Severe Suffering meeting which was held in Gentilly in December. This meeting featured case studies of refinements, guidance on how to use
the RSPCA Roadmap to reduce severe suffering, and the role of Animal Welfare Bodies.
• their 31st
Lay Members' Forum, for members of UK Animal Welfare and Ethical Review Bodies (AWERBs), held in London in December.
Bentley Crudgington and colleagues have written a paper entitled
Creating an effective, inclusive and open Animal Welfare and Ethical Review Body: learning and legacy, which maps the evolution of an Animal Welfare and Ethical Review Body (AWERB) over 7 years and presents a roadmap for other similar bodies.
Laboratory Animals Ltd.
is offering
two scholarships of 2,500 euros each to attend the upcoming
FELASA congress in Athens. The deadline for applications is
25 February.
The COST Action TEATIME has made available an impressive collection of recordings of their
webinars on automated monitoring of behaviour in the home cage of rodents. The collection also includes webinars on other topics such as preclinical models of depression, and the science of communication. This collection is one of many highlighted in Norecopa's
list of recorded webinars, which are also available
sorted by the topics in PREPARE.
Monique Janssens and colleagues describe their plans to
develop a global education hub for animal-free innovation as part of the activity of the
Transition Programme for Innovation without the use of animals (TPI) run by an interdisciplinary cluster of researchers and communication experts in Utrecht.
René Bernard and colleagues have
tested the usefulness of the
EQIPD quality system in preclinical research.
Those who take blood samples from the tail of rodents may be interested in this 3D-printed
mouse tail simulator.
The
Einstein Foundation Award for promoting quality in research recognises contributions that improve the reproducibility and validity of science and research. The submission deadline is
30 April. The Award consists of prize money to the tune of 350,000 euros.
CAAT (the Center for Alternatives to Animal Testing) awards Reduction and Humane Education grants.
Details of the 2024 winners are available on their website. These describe the use of lab-grown human artery replicas, and surgical training on human placentas.
As mentioned previously, the NC3Rs led a study to review the requirements to use animals in WHO guidelines on biologicals. The report from this work, with links to publications,
is available here. In response to this, the WHO produced
a draft guideline on the phasing out of animal tests for the quality control of biological products. The guideline was open for public consultation until 10 January.
The EU Commission is building
a roadmap on the phasing out of animal testing in chemical safety assessments. It has decided to establish a dynamic catalogue of 'transitional initiatives', which are planned courses of action and expected outcomes that contribute to this. The catalogue will be a living document and
made publicly available. They welcome notification of any initiative contributing directly or indirectly to the replacement or reduction of animal use. Notifications can be made by individuals on behalf of any stakeholder or team of stakeholders.
AAALAC has issued a
Position Statement on
The Attending Veterinarian, Veterinary Team, and Provision of Veterinary Care. See also the
ACLAM Guidance on Adequate Veterinary Care.
Students and early career researchers are encouraged to attend a conference in York on 26-27 March entitled
Best Practice in Non-Animal Research Methods.
|
|
The Swedish Research Council (Vetenskapsrådet) has published, in Swedish, their survey in 2024 of
the public's attitude towards animal research.
The Swedish Research Council has been commissioned by the Government to monitor animal testing issues in research, and to provide information about research and research ethics issues, as well as to stimulate debate. The report is part of this work.
54 per cent of the 1 023 survey respondents consider that animal
experiments are acceptable in medical research, and 74 per cent accept them in some contexts. If it is also known that the animals are not exposed to unnecessary suffering, this acceptance increases to 82 per cent.
The attitude of the public has not changed noticeably since the Council’s last previous surveys in 2008 and 2018.
At the same time, the level of knowledge about animal experiments seems to be fairly low. Half of those asked did not know where to turn with questions
about animal experiments. Agencies and universities should be encouraged to increase their communication with the public.
|
|
An excellent series of webinars on the care and use of zebrafish,
the Reporting & Reproducibility series, offers 11 presentations of key issues. All of these webinars have been added to Norecopa's
Webinars & Meetings Calendar, which we invite readers to check regularly for new additions.
Are you aware of
the Nordic Zebrafish Network? This young network has already held two highly successful practical courses for zebrafish users, followed by a scientific meeting. A similar set will be held this Autumn. Please contact
Lars Bräutigam, Karolinska Institutet or
Cesilie Røtnes Amundsen, Nord universitet, for more information. One of the aims of the network, whose foundation Norecopa contributed to, is to share best practice between facilities. In that connection, Cesilie Amundsen and Øivind Torslett from Nord universitet have recently published their experiences in
applying for approval of a zebra fish facility.
Jarl Giske and colleagues propose a new
approach for monitoring and improving the welfare of salmon in captivity, based upon modelling their welfare using "digital twins".
Eleftherios Kasiouras and coworkers have investigated
heart rate monitoring during behavioural stress tests in rainbow trout classified as bold or shy, with the aim of using heart rate as an indicator of stress.
Christine Lavelle and colleagues have written
a review of current research and guidelines for humane killing of laboratory fish, with a focus on fathead minnows.
Mathilde Flueck-Giraud and coworkers have proposed
an adaptable, user-friendly score sheet to monitor welfare in experimental fish.
Swedish scientists have developed a new method for humane
killing of zebrafish employing electric shocks applied directly to the water in which they swim.
We would like to remind fish researchers that there is an increasing number of webinars and courses for these species. A quick search of Norecopa's
Webinars & Meeting Calendar returns 10 events during the remainder of 2025, including
a PhD course in Copenhagen in April.
|
|
Jean-Loup Rault and colleagues have written
a consensus on the definition of animal welfare, to discuss discrepancies in the use of
Positive Animal Welfare (PAW), which 'goes beyond ensuring good physical health and the prevention and alleviation of suffering. It encompasses animals experiencing positive mental states resulting from rewarding experiences, including having choices and opportunities to actively pursue goals and achieve desired outcome'.
The proceedings have been published of
a workshop on animal methods bias, which the authors define as 'a type of peer review bias characterized by a preference for animal-based research methods or lack of expertise to properly evaluate nonanimal methods, which affects the fair consideration of animal-free approaches'.
Contingency planning is an essential part of ensuring the quality, well-being and safety of animal research programmes. The
US National Academies of Science, Engineering and Medicine (NASEM) convened a workshop on the subject in June 2024, and
resources from the meeting are now online.
The UK Home Office has asked the Animals in Science Committee for
policy advice on the content of two important documents: the Non-Technical Summaries of projects and Retrospective Assessments. The aim is to improve openness and transparency, and to support the science sector in implementation of the 3Rs more effectively, in alignment with UK legislation.
Maschi Fabricio and
colleagues share their
experiences in expanding a specific pathogen-free facility, moving from guideline recommendations to practical proposals.
Employees at Sanofi describe how they
use an advisory body on animal ethics to address ethical and societal concerns about animal use.
The European Animal Research Association (EARA) has published the latest edition of their
study of EU-based websites to assess institutional openness in animal research. The findings are also cited
on their website. They also showcase the value of their
Transparency Agreements.
The UK Royal Entomological Society is eliciting
by 1 April respondents to their survey on
the use and ethics of insects in research.
Katerina Stoykova has written
an introduction to the REACH framework and the challenges involved in implementing the 3Rs.
Zeinab Rezaee has proposed
guidelines for animal models of endurance and resistance exercise.
Is there a reproducibility crisis in biomedicine? Kelly Cobey and colleagues
report the results of a survey of 1,600 authors of papers cited in MEDLINE between October 2020 and October 2021. 72% of the participants agreed, with 27% indicating that the crisis was 'significant'. The leading perceived cause of irreproducibility was a 'pressure to publish', with 62% of participants indicating it 'always' or 'very often' contributes.
Lost in translation? Hamideh Frühwein and Norbert Paul discuss animal research in the era of precision medicine.
The journal
Nature Methods describes its routines and
best practice for reporting scientific method, highlighting the
PRO-MaP project (see also
this description from Berlin).
Catharine Krebs and colleagues have published a paper discussing
the risks and costs of solid organ xenotransplantation.
The commercial company Cherry Biotech has produced
Animal Testing Landscape, a description of animal use and alternatives in research.
The
Recombinant Antibodies & Mimetics Database seeks to raise awareness among researchers while encouraging suppliers to adopt innovative and ethically sustainable technologies. It serves as a link between researchers and global suppliers.
Celean Camp of
Replacing Animal Research claims
we must be more social in the debate about potential alternatives to animal use in science.
When will animals be replaced in biomedical research?
Here is EARA's opinion.
Finally, Richard R Rabbit (aka Thomas Hartung) offers tongue-in-cheek advice on
how to be a bad toxicologist, a 'discipline steeped in tradition'.
|
|
Det haster å sende inn nominasjoner til årets 3R-pris.
Fristen er 15. mars. Selvnominasjon er tillatt. Mer informasjon og nominasjonsskjemaet
finner du her.
Den 5. februar arrangerte Dyrevernalliansen
et frokostseminar om regjeringens Dyrevelferdsmelding. Seminaret begynte med korte innlegg om gris, oppdrettsnæringen og forsøksdyr, etterfulgt av en paneldebatt med representanter for Høyre, Venstre, SV, Rødt og MDG. Norecopas sekretær holdt
innlegget om forsøksdyr, hvor han beskrev innholdet i meldingen om forsøksdyr som et tilbakeskritt i forhold til den forrige meldingen fra 2003.
Et opptak av hele det halvannen time lange seminaret
kan ses her eller
lastes ned her.
Referatene fra de to siste styremøtene er blitt publisert siden det forrige nyhetsbrevet.
Vi minner norske lesere om at man kan donere til Norecopa ved å bruke
Vipps, nr.
889149, med et valgfritt beløp.
På forhånd takk for støtten!
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
|
|
|
Please help us in this task by forwarding this newsletter to friends and colleagues who may wish to subscribe. The white box at the bottom right of every page on Norecopa's website, or this link can be used.
|
|
|
Earlier editions of Norecopa's newsletter can be read here. They were published in Norwegian up to no. 2-2017. Free text searches on
Norecopa's website will also find resources which we have described in newsletters.
Mention of an institution, publication or professional service in these newsletters does not necessarily mean that Norecopa endorses all aspects of the activity. Norecopa and its staff are not involved, financially or otherwise, in these external activities unless this is explicitly stated.
|
|
Content:
Norecopa
Editor:
Adrian Smith
Org.no. 992 199 199
Bank account: 2801.53.03931
Vipps: 889149
All photographs in the newsletters have been taken by Norecopa or from
colourbox.com, unless otherwise specified.
You can read about
Norecopa's data protection and privacy policy here.
In compliance with the EU Data Protection Regulation (GDPR), Norecopa updated its personal data and privacy policy in 2018.
You can read about this here.
|
|
|